Oxidation of alcohols
To review, in organic chemistry, an oxidation is essentially making a compound with more carbon-oxygen bonds. The oxidation from an alcohol to a carbonyl is accompanied by the elimination of two hydrogen atoms. An alcohol, with one carbon oxygen bond, can be oxidized to a carbonyl with two carbon-oxygen bonds. If the carbonyl compound is an aldehyde, with two carbon-oxygen bonds, it can be further oxidized to a carboxylic acid, with three carbon-oxygen bonds if a strong oxidizing agent is used. If a weak oxidizing agent is used, it can be oxidized to the aldehyde and stop there.

Oxidation pathway of a primary alcohol
A secondary alcohol can be oxidized to a ketone. Notice the singe carbon-oxygen bond of the alcohol loses two hydrogen atoms and is oxidized to the ketone carbonyl with two carbon-oxygen bonds. There is no need to memorize that secondary alcohols give ketones and primary alcohols give aldehydes, simply remove the two hydrogen atoms from the alcohol and see what you get. Since ketones only have carbon atoms attached to the carbonyl carbon, no further oxidation can occur. Only aldehydes with a hydrogen atom attached to the carbonyl can be further oxidized.

Oxidation pathway of a secondary alcohol
A tertiary alcohol cannot be oxidized. Notice that there are no hydrogen atoms on the carbinol carbon atom. Therefore, two hydrogen atoms cannot be removed from the alcohol, so it cannot be oxidized.

Tertiary alcohols do not oxidize
Oxidizing agents
There are many different oxidizing agents. One strong oxidizing agent is chromic acid. Chromic acid is made from sodium dichromate (Na2Cr2O7) and sulfuric acid (H2SO4).

Chromic acid is such a strong oxidizing agent, that it fully oxidizes primary alcohol all the way to carboxylic acids. It proceeds from primary alcohol to aldehyde to carboxylic acid. Chromic acid oxidizes secondary alcohols to ketones.


But, what if we want to oxidize a primary alcohol to an aldehyde and stop there without going further oxidizing to a carboxylic acid? We need a weaker oxidizing agent. There are several ways to do this, but one is to use a weaker oxidizing agent like PCC, pyridinium chlorochromate. PCC is a complex made from chromium trioxide, pyridine, and hydrochloric acid.

PCC
The weaker oxidizer PCC is strong enough to do the first oxidation of a primary alcohol to aldehyde, but it is too weak to completely oxidize to a carboxylic acid. The oxidation stops at the aldehyde. This is a great way to make aldehydes.

Since PCC is strong enough to do the first oxidation, it is strong enough to oxidize secondary alcohols to ketones as well.

Chromium oxidizers should be handled with care. These chromium salts are hazardous to your health. They should never go down the sink drain in the lab and should be collected as hazardous waste. There are greener oxidizers.
The Swern oxidation or DMP are weak oxidizers like PPC and stop the oxidation of primary alcohols at aldehydes. Instead of chromic acid, bleach can be used as a strong oxidizer.
In fact, aldehydes can be turned into ketones using a little trick. If a Grignard reagent attacks an aldehyde, a secondary alcohol is formed. Upon oxidation, the alcohol turns into a ketone.

1. Fill in the products of the following reactions.
a)
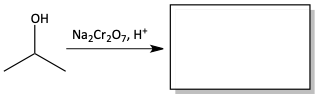
b)

c)

d)

e)
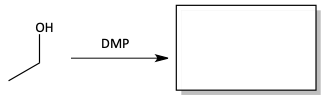
f)

Reduction of carboxylic acid
The opposite of an oxidation is a reduction. The highly oxidized carboxylic acid functional group, with three a carbon-oxygen bonds, can be reduced to an aldehyde.
With a strong reducing agent to alcohol
A strong reducing agent, like LiAlH4, will reduce a carboxylic acid all the way down to an alcohol.

Stopping at aldehyde
If we want to stop at the aldehyde, we need something a little less aggressive. Usually, NaBH4, sodium borohydride, comes to mind as a weaker, gentler reducing agent. But, unfortunately, NaBH4 is too weak to react with carboxylic acids. In fact, we don’t yet have a good way to turn a carboxylic acid into an aldehyde in one step. We must use two steps. There are two common ways to do this.
1. The carboxylic acid can be turned into an acid chloride followed by reduction with tris-tert-butoxylithium aluminum hydride, LiAlH(OtBu)3.

2. A carboxylic acid can be turned into an ester followed by reducing the ester with DIBAL-H to make an aldehyde.

2. Fill in the blank boxes.
a)

b)

c)

Answers
1.
a)

b)

c)

d)

e)

2. Fill in the blank boxes.
f)

a)

b)

c)

