top of page
Carbonyl Synthesis Summary
Reaction Summary
Oxidation of alcohols with weak oxidizer
a)

b)

c)

Swern oxidation and DMP are weak oxidizers like PCC.
Oxidation of alcohols with strong oxidizer
d)

e)

f)

Reduction of carboxylic acid
g)

h)

i)

Alkene/Alkyne oxidations
j)

k)

l)

m)

n)

o)

p)

q)

1,3-dithiane aldehyde/ketone synthesis
r)

Ketones from carboxylic acids
s)

Ketones from nitriles
t)

Carboxylic acids from Grignard reagent and CO2
u)

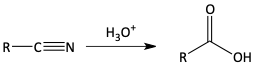
Carboxylic acids from the hydrolysis of nitriles
v)
Carboxylic acids from the permanganate oxidation of alkylbenzene
w)

bottom of page