Lewis Structures
Lewis Structures
Electrons. Chemistry is all about the electrons, those negative charges floating around on the outside of atoms. In fact, the most important electrons in chemistry are those that are on the very outside of the atom. That makes sense because when two atoms approach each other to react, those are the first ones that will bump into each other. These special, outside electrons are called “valence electrons.” The number of valence electrons that surround an atom may be determined by its placement in the periodic table. Hydrogen has one valence electron, carbon has four, nitrogen and phosphorus have five, oxygen and sulfur have six, and the halogens have seven.

Periodic table column numbers showing the number of valence electrons
The normal Lewis dot structures for these atoms have the valence electrons represented as dots around the atom. One electron is placed on each of the four sides of the atom before they are paired.

Valence electrons on organic chemistry elements
The normal bonding pattern for these atoms is with a bond at each site that has only one electron. It will be useful to know the normal bonding patterns of common atoms used in organic chemistry. This is important to aid us in drawing compounds as well as helping us recognize sites in molecules that may be the most reactive.
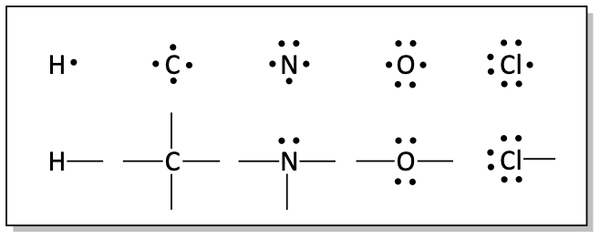
Number of bonds and lone pairs for organic chemistry atoms
Single bonds are not the only types of bonds you will see in organic chemistry. Double (=) and triple (≡) bonds are also commonly found. Because carbon, nitrogen, and oxygen normally form more than one bond, there is more than one normal bonding pattern for each of them.

Common bonding patterns for organic chemistry atoms
There are four different bonding patterns for carbon. This is one of the reasons so many carbon-based, organic, compounds exist.
4. In each of the following compounds, the atoms are drawn, but the bonds and the lone pairs of electrons are missing. Using the normal bonding patterns for these atoms, fill in the bonds and the lone pairs of electrons. I’ve given a few hints in the paragraph after each problem to help you out. Feel free to check your answers at the end of the chapter AFTER you’ve given each one your best attempt.
a)
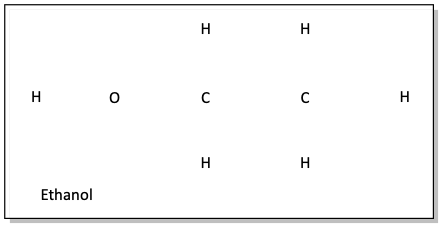
The best way to do problems like this, start off by drawing single bonds to connect all of the atoms.
Then, double check to make sure each atom has the number of bonds it normally has. If more bonds are needed for atoms to be “normal” then consider making double or triple bonds. In problem a, after all of the single bonds are drawn, every atom is “happy”. The oxygen atom normally has two bonds and two lone pairs of electrons. To complete our structure, two sets of electron pairs need to be drawn on the oxygen atom.
b)

After drawing all of the single bonds in problem b, we notice that each carbon atom only has three bonds. Since each carbon atom wants four bonds, we need to make a double bond between the two carbon atoms.

No electron pairs need to be drawn because each carbon atom usually has four bonds and no lone pairs of electrons.
c)

After drawing in all of the single bonds in problem c, we notice that the hydrogens and the first carbon are happy, but the second carbon atom and the nitrogen atom are not. We need to draw in more bonds, but make sure you don’t draw a double bond between the two carbons, or that will make the first carbon have 5 bonds! That is a mortal sin to an organic chemistry professor! So, draw in a double bond between the second C and the N. They’re still not happy. Can you see what we need to do to fix this problem?
d)
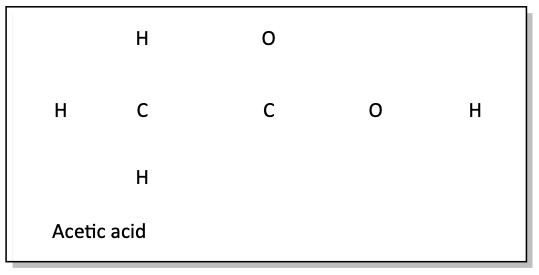
After drawing in all of the single bonds, we find that all of the atoms are happy except the second carbon and the top oxygen. This is a pretty easy fix by drawing in a double bond.
Try these next few problems on your own.
e)
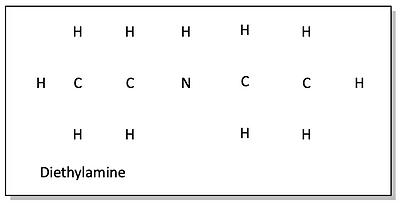



Answers
4. a)

b)

c)

d)
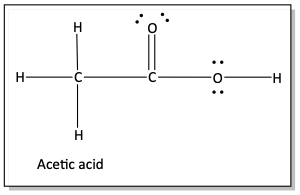
e)

The normal bonding pattern for oxygen atoms is two bonds and two lone pairs of electrons. So, if your answer for peroxyacetic acid had the incorrect bonding patterns for the oxygen atoms, don’t worry about it. We’ll get to it in the next section.