Resonance
Introduction
Organic chemists have agreed to show the movement of electrons by using arrows. A regular, two-headed arrow, <->, shows the movement of two negative electrons. Did you get that? That last sentence may be one of the most important statements in organic chemistry. Arrows show the movement of negative electrons. Arrows DO NOT show the movement of positive charges, atoms, or anything else you can think of. Arrows in organic reaction mechanisms show the movement of negative electrons. Students who fail to learn this are almost never successful in organic chemistry. If you don’t learn to focus on the movement of these electrons, organic chemistry will remain a mystery. It is my belief that successful organic chemistry students learn to think in terms of arrows.
Since opposite charges attract each other, we would expect the following reaction of hydroxide with a positive cation intermediate to proceed as follows.

This is, in fact, one of the products we find. Surprisingly, we also find the following product is made.

By analyzing this surprising product that is made, we can think backward and figure out what unknown cationic or positively charged intermediate could have been attacked by the hydroxide.

Let’s compare these two cationic intermediates we must have had in order to give us these two products.

These two cation intermediates are called resonance structures of each other. Neither one actually exists, but they are convenient representations of the true intermediate. They are useful to us because it makes it much easier for us to explain why a reaction proceeds the way it does.
The symbol we use to signify that two structures are resonance structures of each other is a two-headed arrow, <->. Sometimes, brackets are put around the two structures to identify them as resonance structures.

Resonance Structures
Well, which is it? Is the intermediate the given one, the surprise one, both, or neither? Some people even mistakenly think that these two resonance structures are flipping back and forth from one form to the other. This is not true. The resonance hybrid, the “true” form of the intermediate, is a combination of these two resonance structures. It really looks more like the following.
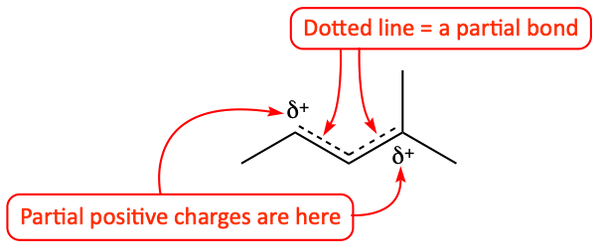
Resonance Hybrid
The two electrons of the pi bond are shared between second and third bond. A solid line drawn between two atoms represents one full, two-electron, bond. A dotted line drawn between two atoms represents a partial bond. So, instead of a double bond at each site, there is really more like 1½ bonds. The one positive charge is shared between the second and fourth carbon atoms. So, instead of having a full positive charge, each of those two atoms carries about ½ of the positive charge. It can get a little confusing. So, in order to simplify our thinking, organic chemists prefer to write the two, distinct, resonance structures.
These two resonance structures are very similar to each other. Notice that the atomic framework in both structures is exactly the same. The only difference between them is the location of the pi bond and the location of the positive charge. If we are given one structure, we can find, or predict, other resonance structures by moving pi electrons around.

Notice, only two pi electrons were moved. The arrow shows the movement of these two electrons, NOT the positive charge. We’ll spend quite a bit of time learning how to find good, valid resonance forms of various structures.
Let’s take a break from this example to discuss resonance structures in more detail. We’ll return and finish up this example later.
Half steps or full steps
Whenever resonance structures are drawn, there are two ways to move electrons. Electrons can move from an atom to an adjoining bond, from a bond to an adjoining atom, or from a bond to a neighboring bond. That may sound a little confusing if you read it too fast. To put it more simply, I use the terminology that electrons can move one full step or ½ step. You won’t find these terms in any other organic text, but I think it makes our discussion of resonance forms more convenient.
Electrons move ½ step if they move from a pi bond to an attached atom or if a set of lone pair electrons moves from an atom to an attached bond. Study some examples of valid ½ steps below.

Examples of 1/2 step movements of electrons
A full step of moving the electrons is moving from a pi bond to a neighboring bond, skipping over the atom between the two bonds.

Example of full step movement of electrons
The reason I call the above pi bond to neighboring bond movement of electrons a “full step” is that it really is the sum of two ½ steps.

Electrons may be moved ½ step or one full step, but they may never be moved farther than one step. This means that electrons are not allowed to move 1½ steps, 2 steps, or more.
1. Each of these problems has an arrow indicating the movement of electrons. Write how many “steps” each movement would be. Then, indicate if the movement is allowed or not allowed by circling the appropriate word.

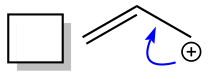
Let’s further investigate how we find good, valid resonance structures. The most important thing is to not forget our mechanism arrow training. It is important to still think in terms of arrows moving electrons. For resonance structures, our arrows will move electrons from one spot to another within the same molecule.
We will do this by moving the electrons ½ step or one full step at a time. Since we are moving electrons, our arrows will always start at a pi bond (in a double or triple bond) or a lone pair of electrons. We can only move these electrons certain places.

Electrons that can be moved in resonance forms
2. For the following structures, put a check in the box if the curved arrow STARTS at a valid location (lone pair of electrons or a pi bond). Put an X in the box if curved arrow STARTS at an incorrect location.
a)


b)
c)

d)

e)

f)
Resonance Rules
There are a few rules for us to consider when we find resonance forms.
1. Don’t start any arrows at single bonds. Your molecule will fall apart if you move single bond electrons. That’s bad.
2. One atom cannot contain more than eight electrons. If you end up with five bonds around a carbon atom, you’ve made a mistake.
3. Electrons cannot move more than one full step.
4. If we have the option, it is better to move the negative electrons towards positive charges (cations) or on to electronegative elements.
5. The molecular structure must remain intact. Atoms are not allowed to be rearranged or moved. Only electrons may be moved around.
6. The sum of the charges on resonance structures must be the same.
Pi electrons towards cations.
Let’s try to find valid resonance forms of the following carbocation.
As we are learning how to do this, it is helpful to draw in all hydrogen atoms. This is sometimes called Grossman’s rule. It is named after chemistry professor Bob Grossman at the University of Kentucky. It can trip us up if we forget how many hydrogen atoms are on each carbon atom. That is easy to mess up for beginning organic chemistry students. Sometimes even experienced organic chemists make this mistake! Grossman’s rule can help us out.
So, let’s rewrite the compound with the hydrogen atoms attached.

Let’s begin looking for a good resonance form. We’ll look at some possible ways electrons could be moved on this compound. We’ll work through several bad attempts until we come to the correct one.
Attempt 1

This is a violation of rule 1. If we move these electrons, we could break our molecule apart. Yikes! A good way to avoid this problem is to ALWAYS start our arrows at pi electrons (from a double or triple bond) or from lone pairs of electrons. In attempt 2, we’ll start our arrow at the double bond (pi bond). We’ll move two of those electrons around.
Attempt 2

This is a violation of rule 5. We rearranged the atomic structure when we were not allowed to change the molecular structure or the arrangement of the atoms. We cannot rearrange the atoms. Notice that there were two hydrogen atoms on the second carbon, but there is only one after the electrons move. There was only one hydrogen atom on the fourth carbon, but there are two after the electrons move.
Attempt 3

Attempt 3 is a ½ step movement of electrons. This is a legitimate way to move electrons. The problem with it is that we get a new resonance structure that has three charges drawn on it. This makes this resonance structure fairly unstable and not very useful. This attempt does follow all of the rules, but it simply takes more experience for us to learn that it is not very useful.
Attempt 4

Attempt 4 is a ½ step movement of electrons, but we simply moved the electrons the other way as compared to attempt 3. Again, it is a legitimate way to move electrons, but not very useful because it makes a triply charged, unstable, resonance structure.
Attempt 5

Attempt 5 is a full step movement of the pi electrons. This movement of the electrons violates rule 2. We have too many electrons around an atom. The carbon atom has the most unfortunate situation of having five bonds! It is also a violation of rule 6. The left resonance form has a +1 charge. The right resonance form has a +2 charge.
Attempt 6

This is a full step movement of pi electrons towards a positive carbocation. A few things should be noted. It is preferred to have negative electrons flow towards a positive site. The atomic structure has not changed from one resonance form to the other. No atoms have more than an octet around them. This is because the positively charged carbon atom only started with six electrons (3 bonds) around it. When the pi electrons moved towards it, it made an octet. The bottom line is a pi bond can move one full step towards a carbocation to make a resonance structure. If the pi bond is further removed from the cation more than one full step, they cannot be pushed towards the cation.

3. One of the following cyclopentene cations has a valid resonance structure and the other does not. For the one that has a valid resonance structure, draw the curved arrow needed and the resonance form. For the one that does not have a resonance form, explain why. It may be helpful to use Grossman’s rule.
a)

b)

4. Draw in the appropriate resonance structures and curved arrow for the following. Pay careful attention to where the positive charge is located in the resonance structures. Grossman’s rule can help you out.
a)

Circle the three atoms on the resonance hybrid of this molecule that have a partial positive charge. You can tell by noticing which atoms in part a have a positive charge drawn on them.
b)

5. The cycloheptatrienyl cation has seven valid resonance structures. Draw in each resonance structure and the appropriate arrows showing how each resonance structure if made. The first arrow has been drawn for you.

Circle the atoms on the cycloheptatrienyl cation hybrid that have a partial positive charge.
Let’s return to our original example and take a closer look at how we would find this new resonance structure. Until we become experts, and even then, it is wise to draw in hydrogen atoms near all of our reactive sites so we don’t lose track of them

Notice that the carbocation only has one hydrogen atom attached to it. All of the other carbon atoms need four bonds.
The carbocation, being positively charged, can be stabilized if some negatively charged electrons can be “pushed” towards it. In our example, we have a pi bond located right next door with plenty of juicy, negative electrons. We can push them over one full step towards the carbocation.

To finish, we need to evaluate our new resonance structure and make sure all of our formal charges are correct. Since our given intermediate has an overall +1 formal charge, we need to have a +1 formal charge in our new resonance structure (rule 6). After a quick analysis, we find that one of the carbon atoms only has three bonds; it must be our carbocation.

This is a correct resonance structure. Let’s look at some things beginning students try to do that are not correct.

Why this is incorrect. Arrows always show the movement of electrons, not positive charges. Never do this. Even if we broke the rules and moved the positive charge, our new resonance form does not make sense. The carbocation should be on a carbon atom with three bonds, not the one with four bonds.

Why this is incorrect. These two arrows give a resonance structure that looks right, but again, arrows always show the movement of electrons, not positive charges. Never do this. Many novice organic chemistry students draw too many arrows for no reason. Do not draw more than one arrow to make a resonance form unless you need to. If an atom gets more than an octet of electrons because a first arrow has moved two electrons onto it, then a second arrow should be drawn to remove electrons. For example, if an atom will have ten electrons, then some electrons need to be immediately removed in order to maintain an octet.
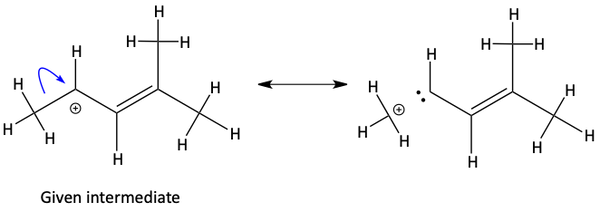
Why this is incorrect. Looking at the resonance structure made from this movement of electrons makes it obvious what the problem is. Resonance structures must keep the same basic atomic structure and only differ by the location of pi and nonbonding electrons. If an arrow starts at a sigma bond, it breaks apart the structure. Never do this.
Pi electrons towards electronegative atoms
We’ve focused on pi electrons moving towards positive cations, but cations are not the only things that pull negative electrons towards them. Electrons are also drawn towards electronegative atoms. Pi electrons can move ½ step onto an electronegative atom if the pi electrons are located adjacent to an electronegative atom like oxygen or nitrogen.
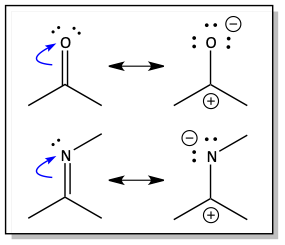
When pi electrons move onto an electronegative atom, we get a resonance structure with a charge separation. We get a positive atom and a negative atom. The electronegative oxygen or nitrogen atom becomes the negative one.
6. In the following reaction, hydroxide is going to react with cyclohexenone. The question is, where? Hydroxide has a negative charge. Since opposites attract, the most positive atoms on cyclohexenone will be where hydroxide attacks. There are two atoms on cyclohexenone which carry a partial positive charge. Let’s try to find them.

Let’s work through it together. First, we notice the electronegative oxygen atom with a pi bond attached. We can move the pi bond ½ step onto the oxygen atom to find one of the partially positive atoms.
a)

This resonance structure has a pi bond adjacent to a positively charged carbon atom (carbocation). Move some pi electrons one full step towards the carbocation.
b)

Now, since we have two resonance structures with positively charged atoms, we have insight into the reactivity of cyclohexenone.
c) Circle the two atoms on cyclohexenone hybrid that have a partially positive charge.

7.
a) Find resonance forms to help find the three atoms on the following compound that have a partially positive charge. Draw in all appropriate arrows and resonance forms.
b) Circle the three atoms that are partially positive.

8. Four atoms of benzaldehyde have a partial positive charge. Draw resonance forms to find them all.
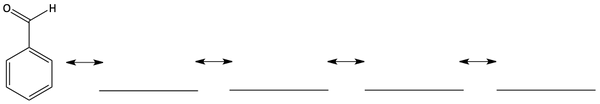
Circle the four atoms on benzaldehyde that have a partial positive charge.
Lone pairs moving towards pi bonds
Pi bonds are not the only electrons that can be moved to make resonance forms. A lone pair of electrons (especially at a position with a formal negative charge) is a great place to start pushing electrons. They can be pushed ½ step IF AND ONLY IF a situation does not occur where an atom ends up with greater than eight electrons on it (an octet). An atom can temporarily have more than eight electrons around it, IF DURING THE SAME STEP, electrons are removed from the back side of said atom to correct the problem of having too many electrons.
Let’s take a closer look to see what I mean. Consider the following anion.

If I push the lone pair from the negative site ½ step, we run into a bad situation.

We have a carbon atom with five bonds! That’s bad unless we can fix the situation by moving some electrons away from it. But, in this example, the problem cannot be fixed because all of the bonds on the backside of this carbon are all single bonds and cannot be moved away.

We cannot start arrows at single bonds to make resonance forms because our molecule will fall apart.
How about the following carbanion?

We move the lone pair of electrons from the negative site ½ step and see what we get.

Again, we get the bad situation of having 5 bonds around a carbon. We have more than eight electrons around the carbon and have broken the octet rule. But, don’t panic just yet. We’ll be OK if we can move electrons away from this overloaded carbon atom. In this case, we have a pi bond that can be pushed away ½ step onto another carbon atom to remove some electrons. We’re saved!

Normally, organic chemists do not draw the bad looking structure with five bonds around the carbon. We know that it is not a good structure, so we don’t want to pretend that it is valid in the least. So, we typically draw both arrows on one structure like this.

9. Finish the movement of the electrons on this structure and draw the resonance structure made

This a very good resonance structure because electrons end up on an electronegative oxygen atom!
10. Nitropropane has one other good resonance structure. Draw it.

Since the two oxygen atoms share 1 negative charge, the resonance hybrid (or the true structure of the molecule) has ½ of a negative charge on each oxygen atom.
11. The cyclopentadienyl anion has five valid resonance structures. Draw them and the appropriate arrows.

Circle the atoms on the cyclopentadienyl anion that have a partial negative charge.

12. The phenoxide anion has four atoms with a partial negative charge on them. One is the oxygen atom which is clearly delineated in the given structure. Draw three other resonance forms to find the other three negatively charged atoms.
Circle the atoms on the phenoxide anion that have a partial negative charge.

13. Draw in the two resonance forms as well as all appropriate arrows to show the movement of electrons in the following.
a)

In this new resonance structure, we can push the carbanion electrons towards the carbonyl C=O. Draw in the second arrow needed and then the resonance structure made.
b)

We can put it all together and get the following.
c)

Circle the three atoms that are partially negative.
d)

14. The following movement of electrons is not allowed in order to make a new resonance form. Why?

15. The following compound does have two valid resonance forms. Draw them and appropriate arrows.
a)

b) Circle the three atoms that are partially negative.

Answers
1.

2.

3.
a)

b)

4.
a)
b)
5.

6.
a)

b)


c)
7.
a)
b)
8.
a)

b)
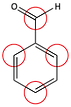
9.

10.

11.


12.


13.
a)

b)

c)

d)

14.

15.
a)

b)