Base Strength
Which is the better base?
A base is a compound that grabs a proton, H+. If we are asked the question, “Which of two compounds is the better base?” we are really being asked, “How likely is the compound to go and grab a proton, H+? If the compound is very happy, it will stay the way it is and not grab a proton. It would be a poor base. If it is not happy, it is more likely to go grab an H+ and be a good base. Another way to think about it is a good base has the most concentrated negative charge and is most likely to go grab an H+.
For the following, the strongest base of each pair is circled.



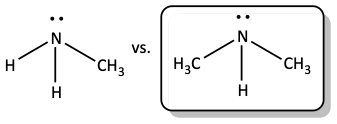


9. In each of the following pairs, circle the one that is the stronger base.
a)
b)
c)
d)
e)
10. What is the conjugate base, if any, for the following compounds?
a)
b)
c)
d)
e)
11. The butyl anion (CH3CH2CH2CH2:-) is a much stronger base than bromide (Br-). Explain.
Answers
9.
a) The lone pair of electrons are more spread out on the larger P atom than the N atom making the electron density more concentrated on the N and ready to go react.
b) The negative charge is spread out more on the Br- (larger) than the F-.
c) Both N and O are in the same row. O is more electronegative than N so is more stable with the negative charge making the NH2- the stronger base.
d) Same row. Cl is more electronegative and more stable with the negative charge than S.
e) The alkyl groups donate electron density towards the negative charge. Isopropoxide has more alkyl groups donating electron density making its negative oxygen less stable and more reactive, more basic.
10.
11. The butyl anion (CH3CH2CH2CH2:-) is a much stronger base than bromide (Br-). Explain.
The negative charge on the carbon of the butyl anion is on a smaller, carbon atom. It is more localized. The negative charge is more spread out on the bromide ion since it is larger. This makes it more stable, happier. Therefore, the butyl anion is more likely to grab a proton, making it a stronger base.