SN2 Reaction - Backside Attack
Reactions of Alkyl Halides
Consider the molecule, bromomethane. The halide of bromomethane is more electronegative than the carbon atom to which it is attached. This makes the carbon atom partially positive, so a negatively charged nucleophile will attack it. This is the basis of the reactivity of alkyl halides.

There are four reactions for alkyl halides that we will be learning about for the remainder of this chapter. They come in two main types, substitutions and eliminations.
Substitutions
Substitutions are when one group replaces another, much like when a substitute teacher replaces a regular teacher who is absent from school.

Generic substitution reaction
Eliminations
When something is eliminated, that means something is removed or expelled. When we eliminate waste from our bodies, we remove or expel the waste. In organic chemistry, eliminations are two groups being removed from a compound (H and X in this case) forming a π bond.
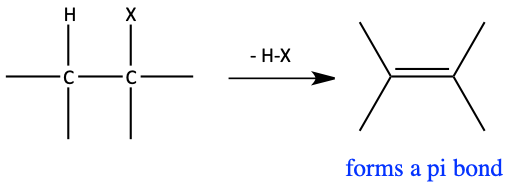
Generic elimination reaction
7. Label each of the following reactions as either a substitution or an elimination.
a)

b)

c)

d)

e)

The SN2 Reaction - The "backside attack"
Let’s take a closer look at the substitution reactions first. There are two main mechanisms for the substitution of an alkyl halide. The first substitution mechanism we will look at is called the SN2 mechanism. Let’s walk through the SN2 mechanism by analyzing the reaction of two species, a hydroxide ion and bromomethane.
In this substitution, an aggressive nucleophile attacks the carbon atom of the alkyl halide from the back side opposite the halide. If this nucleophile makes a bond with the carbon atom, that would make five bonds on the carbon atom! This cannot happen, so a group needs to leave. As the “backside attack” happens, the halide leaves. The halide is called a leaving group. The SN2 reaction all happens in one step.
This is an example of a typical SN2 reaction.




Example of SN2 reaction
If you wanted to do a reaction with these two species, you could buy them from a chemical company. You would be able to find bromomethane in the chemical catalog, but you would not find hydroxide. That is because hydroxide does not exist by itself because of its negative charge. It exists with a positive counterion. You would need to buy sodium hydroxide (NaOH) or potassium hydroxide (KOH). But, in the reaction, the alkali metal is not involved in the reaction. It is called a spectator ion. A spectator ion is not involved in the reaction. It just floats around and acts like a spectator. It watches. Therefore, at times, the spectator ions are not explicitly drawn in our reactions. Spectator ions are sometimes ignored.
So, why the weird name, SN2?
S: Substitution. This is a substitution reaction.
N: Nucleophilic. A nucleophile is attacking an alkyl halide.
2: A Bimolecular mechanism. Two species are involved in the rate-determining step.
The substitution and nucleophilic part are self-explanatory. But, what does the bimolecular part mean? The SN2 reaction mechanism is a one-step mechanism. It is a concerted mechanism, meaning the steps happen at the same time. The nucleophile attacks at the same time as the halide leaving group leaves.

Reaction coordinate diagram of an SN2 reaction
This one step is also the slowest step in the reaction because it is the only step! In this slowest step, the rate-determining step, two molecules are involved, the hydroxide and the bromomethane. For the SN2 reaction to occur, these two molecules need to meet each other. If the amount of hydroxide were doubled in the reaction, what would happen to the odds of the two colliding? It would double, or it would double the rate of the reaction. If the amount of bromomethane in the reaction were doubled, what would happen to the odds of the two colliding? It would double, so it would double the rate of the reaction. The reaction is first order with respect to each reactant and is, therefore, 2nd order overall. The rate equation for the SN2 mechanism is:
Rate = kr[Nuc][Substrate]
or
Rate = kr[OH-][CH3Br]
Factors that affect the SN2
Why a backside attack?
One consideration is sterics. A frontside attack of the nucleophile is blocked by the halide leaving group. Another consideration is electronics. The leaving group is negatively charged and the nucleophile is typically negatively charged. There is an electronic repulsion between the two preventing the nucleophile from attacking from the front side. A backside attack is therefore what happens.

The Substrate
A nucleophile attacks a substrate (the alkyl halide) in an SN2 reaction. For the SN2 reaction, since the substrate is attacked from the backside by the nucleophile, the steric hindrance to that backside attack is important. In order for the nucleophile to be able to reach the carbon atom, it needs to navigate past three groups pointing towards the backside of the substrate. If these groups are small, like hydrogen atoms for a methyl halide, the nucleophile can more easily reach the carbon atom. If there is one alkyl group (primary halide), it still happens pretty easily. With two alkyl groups (secondary halide), it becomes more difficult. With three alkyl groups (a tertiary halide), it is nearly impossible for the nucleophile to reach the carbon atom. This is steric hindrance. The alkyl groups play defense on the backside and prevent the nucleophile from reaching the carbon atom.
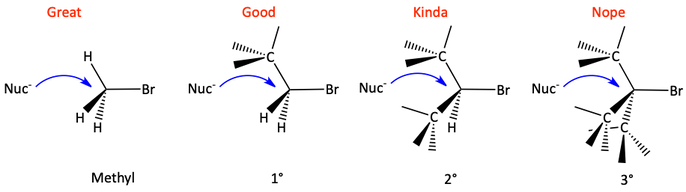
The backside attack of an SN2 reaction
In general, the rate of reaction of the substrates in an SN2 reaction is:
Me > 1° > 2° >>> 3°
The rate of reaction for an SN2 reaction
The substrate does not need to be tertiary for the backside attack to be sterically hindered. For example, neopentyl bromide, a primary alkyl halide, is very slow to the SN2. It is as bad as most tertiary alkyl halides.


The Leaving Group
In an SN2 reaction, the leaving group usually has a negative charge on it. Better leaving groups are those that are happier and more stable with the negative charge. Nature abhors an isolated charge, so it is better the more the negative charge can spread out. For halide leaving groups, the larger atoms (lower in the periodic table) are more stable because they can spread out the negative charge more. They make better leaving groups.
I- > Br- > Cl- > F-
F- is a poor leaving group because it is so small. Since oxygen and nitrogen are in the same row of the periodic table, O- and N- are also small. They are less stable than F- because they are less electronegative than F. Therefore, HO-, RO-, and H2N- are poor leaving groups. Spreading the negative charge through resonance is the only way to make O- a good leaving group. Resonance is why the following are excellent leaving groups. In mesylates and tosylates, the negative charge is spread over three oxygen atoms. Mesylates and tosylates can be made from alcohols as we will learn later.


The order of leaving groups stability is

Stability of leaving groups
The Solvent
The solvent that is chosen for an SN2 reaction can either speed up the reaction or slow it down. Polar solvents are generally used to dissolve the reactants in an SN2 reaction since ions are generally involved as the nucleophile and leaving groups. The polar solvent used can be either a polar protic solvent or a polar aprotic solvent.
Polar Protic Solvent
Polar protic solvents are those with protons (hydrogen atoms) attached to an oxygen or nitrogen atom. The protons are small, concentrated centers of positive charge. They, therefore, attach themselves to the negatively charged nucleophile. The nucleophile is solvated, meaning the solvent surrounds it. Therefore, the nucleophile is surrounded and has difficulty attacking the carbon atom of the substrate. The reaction proceeds very slowly.

Polar Aprotic Solvent
Polar aprotic solvents are those with no O-H or N-H bonds. Examples are:




Polar aprotic solvents
Polar aprotic solvents are polar enough to dissolve the substrate and the nucleophile, but they do not solvate the nucleophile. In fact, the solvent surrounds the positive counterion (Na+ or K+) instead. Since the cation is bound up by the solvent, it cannot attach to the nucleophile. This makes the nucleophile extra negative and very strong. Polar aprotic solvent speed up an SN2 reaction. Most SN2 reactions are therefore performed in polar aprotic solvents. For example, if KOH is added to bromomethane in acetone, the following occurs.

The Nucleophile
For an SN2 reaction, the nucleophile is aggressive. The nucleophile must attack the substrate before anything else happens. So, what makes a strong, aggressive nucleophile? Remember, nucleophiles are lovers of nuclei (which are positively charged). Therefore, nucleophiles are species that attack positive charges. In this case, a good nucleophile will attack the carbon atom of the substrate that has a partially positive change. One thing that makes a nucleophile aggressive is if it has a negative charge on it or a great big cloud of negative electron density. The hydroxide ion is an excellent nucleophile because it is negatively charged.
We need to define a couple of terms since negatively charged species act as both nucleophiles and bases.
Nucleophilicity is a measurement of how much a species wants to attack a positively charged atom (typically an electrophilic carbon atom).

Basicity is a measurement of how well a species abstracts a proton (H+) by donating its electrons to it.

In the SN2 reaction, we need a good, strong nucleophile. So, what makes a good nucleophile?
Negative charge helps.

Remember that anions exist in their salt form, so many times they are written with their positively charged cationic counterions attached. Look for metals like Na, K, or Li with them.

Down a column helps.
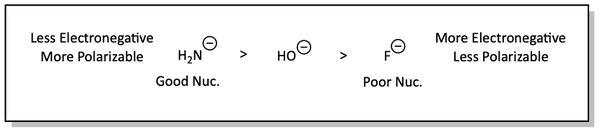
Nucleophilicity increases for atoms down a column in the periodic table. This is because these atoms are larger. We say that they are more polarizable or softer. I like to think of them as big, soft, fluffy, squishy electron clouds. These large, squishy electron clouds can reach out their electrons and squeeze around the backside alkyl groups more easily to reach the carbon atom of the substrate. So, nucleophiles are better if they are softer or more polarizable.

Across a row hurts.
This is not as big of an effect, but nucleophilicity decreases across the atoms in a row or period in the periodic table. This is because, across a row, the atoms become more electronegative and hold onto their electrons more. Since they hold their electrons stronger and closer to themselves, they are less polarizable, harder, or less squishy. They cannot reach out their electrons as well to the carbon atom of the substrate.
Resonance hurts.
Nucleophiles are less likely to go out and attack the substrate the more stable they are. Good nucleophiles are unhappy, negatively charged species that want to give away that negative charge. So, if resonance forms can be drawn to spread out a negative charge or a lone pair of electrons on a nucleophile, it becomes more stable. The attacking atom has less negative electron density on it and is less likely to go attack a positive carbon atom. It is then a worse nucleophile.

Inductive effects
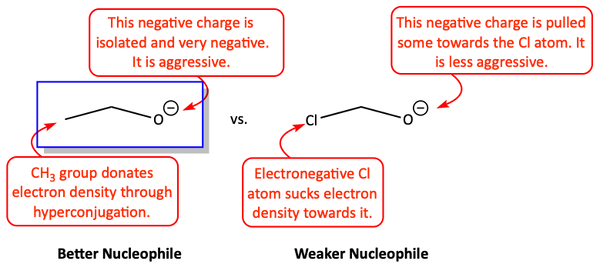
Resonance is not the only way to spread out a negative charge. Negative charges can also be spread throughout a molecule through induction. If an electron-withdrawing group is on the nucleophile, it can pull electron density away from the attacking atom.
Sterics hurts
Large, polarizable attacking atoms increase nucleophilicity because the electron cloud can squeeze and reach out to the carbon atom of the substrate. But, it is different if the nucleophile is large because of attached alkyl groups. These groups do not increase the polarizability of the attacking atom, rather they make the nucleophile big and bulky. For example, t-butoxide is so large, the negative oxygen atom cannot reach the carbon atom of the substrate.


Large, bulky anions like t-butoxide are poor nucleophiles, but they do have a negative charge that will still aggressively attack positive charges. t-Butoxide will grab a proton (H+) quickly making it a good base. It can do this because protons are typically on the outside of a substrate where they can be easily reached, unlike the carbon atom.
Stereochemistry - inversions
When the backside attack happens in the SN2 mechanism, the substrate begins to open up in the transition state and then flips inside out or inverts. This is called a Walden inversion. This often flips the stereochemistry at the substrate’s carbon atom. In this example, an (S) configuration flips to an (R) configuration. Since there is always an inversion in the SN2 reaction, it is a stereospecific reaction. The same stereochemistry changes always occur. One stereoisomer reacts to give one specific stereoisomer of a product.


Anionic vs. Neutral Nucleophiles
We have seen that nucleophiles can be both anionic and neutral. They have very slightly different mechanisms. If an SN2 reaction involves a neutral nucleophile, it makes a positively charged product. Typically, there is a cleanup step at the end where a proton (H+) is removed.

When ammonia, NH3, was the nucleophile in the SN2 reaction, it made a positively charged ammonium salt. Another molecule of ammonia acted as a base to deprotonate the ammonium salt making a neutral amine.
Some common nucleophiles
All of the strong nucleophiles have a negative charge, are fairly large, or have a lone pair of electrons on nitrogen or phosphorus. The weak nucleophiles are neutral molecules that are not nitrogen or phosphorus bases, or they have a negative charge on a very electronegative element like F, or resonance stabilized like acetate.
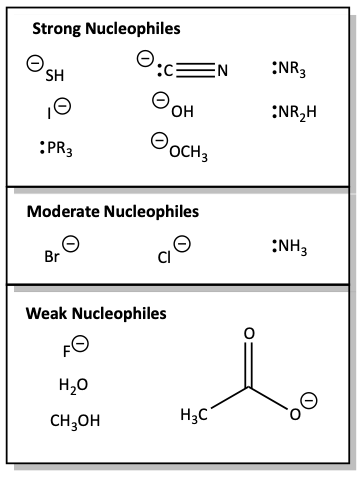
Strength of common nucleophiles
SN2 Examples
The SN2 reaction is a powerful reaction. We can use it to produce many different types of compounds from an alkyl halide.

Example SN2 reactions
Why SN2 reactions do not occur on sp2 carbon atoms
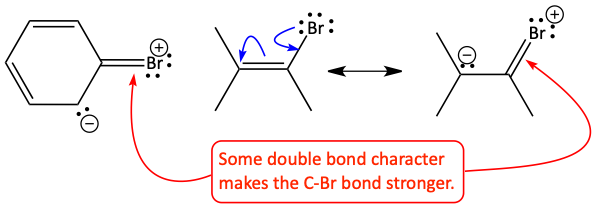
We also do not see SN2 reactions occur on sp2 carbon atoms because the backside attack is prevented. If the halogen is on a benzene ring (an aryl halide) or an alkene (a vinyl halide) SN2 reactions do not occur. There are a couple of reasons for this. Two of the simpler explanations are: 1. the electron cloud of the aromatic ring or alkene prevent the negative nucleophile from reaching the carbon atom. 2. The carbon-halogen bond is stronger than usual. It is more double bond like because of resonance forms that can be drawn.
Reason 1



Reason 2

8. Explain why neopentyl bromide, even though it is a primary alkyl halide, does not do an SN2 reaction.
9. Rank the nucleophilicity of OH-, H2O, CH3O-, and CH3COO-.
10. Rank the nucleophilicity of NH3 and PH3.
11. Give the organic product in the following substitution reactions.


Answers
7.
a)

b)

c)

d)
e)

8.
Even though it is a primary alkyl halide, neopentyl bromide is still a sterically hindered substrate preventing the backside attack SN2 reaction from happening.

9.
CH3O- > OH- > CH3COO- > H2O
Strongest…………… ……….weakest
Methoxide (CH3O-) has electron donating methyl group on it making the negative oxygen atom very negative. The hydroxide (OH-) oxygen has a negative charge on it, but the hydrogen atom doesn’t donate in the electron density like the methyl does, so the oxygen is not as negative. The acetate (CH3COO-) has a resonance form making each oxygen atom carry half a negative charge. The water is not negatively charged at all so the lone pair of electrons will attack, but water is very stable and not eager to do so.
10.
PH3 is a stronger nucleophile than NH3 because P is larger than N and is softer and more polarizable. It can donate its electron density into the substrate.
11.
Give the organic product in the following substitution reactions.
