SN1
The SN1 Reaction
Unlike the SN2 reaction that is one-step, the SN1 reaction occurs in several steps. The first step of the SN1 reaction is the leaving group leaving the substrate to make a carbocation. Once the carbocation is formed, a nucleophile attacks it. If this nucleophile is neutral, it makes a protonated, positively charged species. This species is deprotonated (usually with a solvent) to make the product.

Generic SN1 Reaction Mechanism
S: Substitution. This is a substitution reaction.
N: Nucleophilic. A nucleophile is attacking an alkyl halide.
1: Unimolecular. One species is involved in the rate-determining step.
The SN1 reaction is at least a two-step reaction mechanism. The reaction energy diagram is below. The first step is the slowest, rate-determining step (R.D.S.). The second step is a fast step. In a two-step reaction energy diagram, there are two transition states and one intermediate. The intermediate, in this case, is a carbocation.

Reaction coordinate diagram for an SN1 reaction
The S and N of this mechanism’s name are because the SN1 reaction is a nucleophilic substitution. This is obvious. The 1 in the name is because it is unimolecular (involves one molecule) in the rate-determining step. The first step of the SN1 reaction is the slowest step. The reaction cannot proceed until the leaving group leaves. In step 1, notice that there is only one molecule involved, the alkyl halide, R-X. If more nucleophile is added to the reaction (water in our example), the reaction will not go any faster. The extra water molecules will stand around waiting for a carbocation to be formed. They cannot do anything until that happens. But, if more alkyl halide, R-X, is added to the reaction, the reaction will go faster because the carbocation will be made faster. Therefore, the reaction is first order with respect to R-X and zeroth order with respect to the nucleophile. The SN1 reaction is first order overall.
Rate = kr[Substrate]
or
Rate = kr[R-X]
The Substrate
The SN1 reaction makes a carbocation. The happier or more stable this carbocation is the easier it will be for the halide to leave. Therefore, the reaction goes faster. So, what makes a good carbocation?
At a first glance, we look for the substitution of the carbocation atom. We remember that tertiary carbocations are the most stable because of hyperconjugation. Primary and methyl carbocations very unstable. Therefore, tertiary alkyl halides make excellent substrates for the SN1 reaction, secondary carbocations are OK but primary and methyl alkyl halides cannot undergo SN1 reactions unless something else is involved. This is the opposite of what we saw in the SN2 reaction.


The carbocation substrate of an SN1 reaction
The other thing to look for in regards to the stability of the carbocation is resonance. Both allylic and benzylic carbocations are quite stable because resonance forms can be drawn. Allylic carbocations are carbocations that neighbor a double bond. In allylic carbocations, the nucleophile can attack either carbocation spot to make different products. Benzylic carbocations are carbocations neighboring a benzene ring. In benzylic carbocations, the only resonance form the nucleophile attacks is the one with the carbocation next to the benzene ring, not the with the carbocation on the benzene ring.
Allylic carbocations

Benzylic carbocations



8. Are the following substrates more likely to undergo a substitution reaction via SN1, SN2, or both?
a)
b)

c)

d)

9. Indicate if the following substrates would form a resonance-stabilized carbocation after the leaving group leaves.
a)

b)

c)
d)

The Leaving Group
Like in the SN2 reaction, good leaving groups must be stable after they leave. One small difference is that in the SN1 reaction, sometimes the leaving group is a neutral molecule. Often, this happens when a reaction is run under acidic conditions. For example, the hydroxyl group of an alcohol is a poor leaving group. But, in acidic conditions, the hydroxyl group can be protonated and converted to water, which is a very good leaving group.



10. Label the leaving groups on the following compounds as good or bad.
a) PhCH2Cl
b)

c)

d)

The Nucleophile
Since the first step of the SN1 reaction is the leaving group leaving to make a carbocation, the nucleophile in the SN1 is typically a weak, neutral molecule. We do not want an aggressive nucleophile that attacks right away, but we want a nucleophile that will wait around until after the leaving group leaves. Also, if the nucleophile is a negatively charged base, it will perform an elimination reaction before allowing the SN1 to proceed. In an SN1 reaction, the nucleophile is often the solvent. SN1 reactions are called solvolysis reactions because of this.




11. Identify whether the following nucleophiles are more likely to be involved in an SN1 or an SN2 reaction.
a)
b)
c)
e)
d)
The Solvent
The first step in the mechanism is the formation of a positive carbocation and a negative leaving group, which are ions. The formation of these ions is helped by the presence of a polar solvent that can surround the ions and help stabilize them. Polar solvent molecules have a positive end, which can surround, attach to, and help stabilize the negative leaving group. The polar solvent molecule also has a negative end, which can surround, attach to, and help stabilize the positive carbocation. Nonpolar solvents cannot attach to the ionic species and therefore do not help stabilize or lower their energy. SN1 reactions prefer polar solvents.
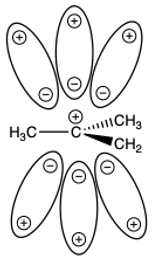

The Stereochemistry
The carbocation intermediate is a flat, planar species. Because it is flat, the nucleophile can attack the empty p-orbital from the topside of the intermediate or the bottom side of the intermediate. This leads to two different products, the retention product if the nucleophile attacks the same side as where the leaving group left and the inversion product if it attacks from the backside of where the leaving group left. This leads to a racemic mixture of products if there are three different groups on the carbocation leading to chiral molecules.

Formation of a racemate in the SN1 reaction
Rearrangements
Any time a carbocation forms, like in SN1, it is possible that a side-reaction called a rearrangement may occur.
1,2-Hydride Shift
Let’s look at the simplest of the rearrangements, the 1,2-hydride shift, and see why it happens. In this SN1 reaction, a secondary carbocation is formed after the halide leaves. At this point, the nucleophile could come in and make the product. But, in this particular case, a rearrangement can happen. The carbocation wants electrons. A hydride, a hydrogen atom with its two electrons, moves from a neighboring carbon to the carbocation. This is called a 1,2-hydride shift. Why would this happen? What is the driving force? In this particular case, once the hydride shifts over, a secondary carbocation becomes a more stable tertiary carbocation. This tertiary carbocation is happier, lower energy, and is preferred. If more stable carbocations are formed, a rearrangement may occur.

The 1,2-hydride shift rearrangement
Usually, at this point, a sense of panic starts to set in on the part of the typical student. They begin to wonder how in the world they will be able to predict when this will happen. Don’t worry too much about that. Often, you will not be asked to predict when a rearrangement will happen. Instead, you will more often be asked to identify, after the fact, when a rearrangement has happened. This is much easier to do. It is often an indication of a rearrangement if you ever notice that the carbon framework has changed.
1,2-Methyl shift
This is not as big of an effect, but nucleophilicity decreases across the atoms in a row or period in the periodic table. This is because, across a row, the atoms become more electronegative and hold onto their electrons more. Since they hold their electrons stronger and closer to themselves, they are less polarizable, harder, or less squishy. They cannot reach out their electrons as well to the carbon atom of the substrate.

The 1,2-methyl shift rearrangement
Ring expansion
Larger alkyl groups than methyl can also shift. In this example, the alkyl group that shifts is a longer chain that wraps around and hooks onto another part of the molecule. The cyclopentyl group opens up as the CH2 does a 1,2-shift. Therefore, the ring gets one carbon larger becoming a cyclohexyl ring. This ring expansion rearrangement is usually the trickiest rearrangement, so study it until you clearly understand it.

The ring expansion rearrangement
12. CH3CH2OH does not react with NaBr, but adding H2SO4 forms CH3CH2Br. Explain.
13. The primary alkyl halide, CH2=CHCH2Cl, undergoes an SN1 reaction faster than the secondary alkyl halide, (CH3)2CHCl. Explain why.
14. Explain why PhCH2Cl is hydrolyzed (reacts with water) slowly to form PhCH2OH, but forms the alcohol quickly if a small, catalytic amount of potassium iodide, KI, is added to the reaction.
15. Explain why the following bicyclic alkyl halide does not undergo SN1 or SN2 reactions.

16. Label the following reactions as more likely to react via an SN1 or an SN2 mechanism.
a)

b)
c)


d)

Answers
12. CH3CH2OH does not react with NaBr, but adding H2SO4 forms CH3CH2Br. Explain.
The OH group of CH3CH2OH (ethanol) is a bad leaving group. Therefore, the Br- nucleophile of NaBr cannot attack the ethanol. Adding H2SO4 will protonate the OH group of the ethanol making it water, –OH2+. This water leaving group is a great leaving group which now allows the Br- nucleophile to attack the ethanol and the water leaving group leave making ethyl bromide, CH3CH2Br.
13. The primary alkyl halide, CH2=CHCH2Cl, undergoes an SN1 reaction faster than the secondary alkyl halide, (CH3)2CHCl. Explain why.
Once the leaving group leaves the alkyl halide alkene, a resonance-stabilized carbocation is formed.

The secondary alkyl halide will make a secondary carbocation, not bad, but not as good as spreading out the positive charge over two carbon atoms.
14. Explain why PhCH2Cl is hydrolyzed (reacts with water) slowly to form PhCH2OH, but forms the alcohol quickly if a small, catalytic amount of potassium iodide, KI, is added to the reaction.
The I- from KI can attack the PhCH2Cl by an SN2 reaction to make PhCH2I. I- is a better leaving group than Cl-. This makes the reaction with water to form PhCH2OH faster.
15. Explain why the following bicyclic alkyl halide does not undergo SN1 or SN2 reactions.
Because it is a bicyclic compound, it cannot do a Walden inversion for an SN2 reaction. It is also sterically hindered and too crowded for a backside attack. Because of the bridges, it also has difficulty becoming a planar carbocation in an SN1 reaction.
16. Label the following reactions as more likely to react via an SN1 or an SN2 mechanism.
a) SN2 Secondary alkyl halide substrate could go either way. Hydroxide nucleophile is aggressive.
b) SN1 Tertiary alkyl halide is too crowded for SN2 and will stabilize the carbocation of SN1. Water is a weak nucleophile.
c) SN1 OH is a poor leaving group. The acid can protonate it making water which is a good leaving group. It is tertiary which makes a good carbocation (SN1). The chloride nucleophile from the HCl will then attack.
d) Neither, OCH3 is not a good leaving group.