E1and E2
Beta-Elimination reactions (E1 and E2)
In beta-elimination reactions, a base reacts with a substrate (like an alkyl halide) to make a π-bond. Two groups are removed or eliminated from the substrate. One is a hydrogen atom and one is a leaving group. The carbon atom attached to the leaving group is the alpha carbon. There must be a hydrogen atom on an adjacent carbon atom (a beta carbon atom). The molecule cannot eliminate if there is no beta hydrogen atom. There are two types of elimination reactions, the E1 and the E2.

A generic elimination reaction
The E1 reaction
The E1 reaction is an elimination that is unimolecular. It is unimolecular because there is only one species, the substrate, which is involved in the rate-determining step. The rate of the reaction depends on the rate of the leaving group leaving the substrate. The amount of base in the reaction has nothing to do with that.
E: Elimination. Two groups are eliminated to form a π-bond.
1: Unimolecular. One species is involved in the rate-determining transition state.
The rate of the E1 reaction only depends on the concentration of the alkyl halide substrate. It does not matter how much of the base is in the reaction.
Rate = kr[Substrate]
or
Rate = kr[R-X]
The mechanism for the E1 reaction is as follows.

An E1 mechanism
The first step of the E1 reaction is the same as the SN1 reaction. First, the leaving group leaves to form a carbocation. Once a carbocation is formed, the base removed a beta-hydrogen atom to form the π-bond.
The E1 mechanism competes with the SN1. Once a carbocation forms, the weak nucleophile can attack the alpha carbon to do SN1 or it can act as a base and remove a beta-hydrogen to do E1.

The E1 mechanism competing with the SN1 mechanism
Since the first step of an E1 reaction is to form a carbocation, do you think the E1 reaction could have rearrangements? Of course it could. This is something for us to watch for.
The E2 reaction
The E2 reaction is a concerted reaction. All steps happen at the same time. An aggressive base pulls a proton (H+) off of a beta carbon. At the same time, the electrons between the proton and beta carbon atom form a π-bond at the same time as a leaving group leaves the alpha carbon. Since this is a concerted, one-step mechanism, there is only one transition state. In this transition state, which must be the rate-determining step, two species are involved, the base and the substrate. This is why there is a “2” in the E2 name.
E: Elimination. Two groups are eliminated to form a π-bond.
2: Bimolecular. Two species are involved in the rate-determining transition state.

A generic E2 mechanism
Since two species are involved in the rate-determining step, the rate equation for the E2 reaction is:
Rate =kr[Base][Substrate]
Nucleophiles and Bases
A base grabs an H+ (think E2). A nucleophile attacks a carbon atom (think SN2).
The E2 reaction typically requires a strong, aggressive base. The deprotonation needs to occur immediately. If the leaving group leaves first, it is an E1 or SN1 mechanism.
Strong Nucleophile/Strong Base
What makes a strong, aggressive base? Strong bases often have negative charges like HO-, RO-, NH2-, etc. These are both good bases and good nucleophiles.
Some strong bases won’t eliminate but will substitute instead. A good, small base like HO- or CH3O- may SN2 backside attack an alkyl halide that is not crowded instead of performing an E2 reaction. In fact, the SN2 reaction is favored under these conditions.

E2 competes with SN2
Strong Nucleophile/Weak Base
Sometimes, though, a negatively charged species is a strong nucleophile while being a weak base. It is a weak base if the negatively charged species is pretty stable. Examples of strong nucleophiles that are weak bases are negatively charged halides or when a negative charge can be spread out on the species using resonance forms. These are poor bases because they don’t want to grab an H+. But, because they are large and polarizable, they are good nucleophiles and can attack a carbon atom in an SN2 reaction.
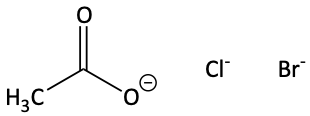
Strong Base/Weak Nucleophile
Using a bigger, bulkier base is one way to get more of the E2 reaction to occur over SN2. For eliminations, it is better to use the larger t-butoxide (-OtBu) instead of using the smaller CH3O-, methoxide. Big, bulky bases like t-butoxide are much worse nucleophiles. They have difficulty reaching the alpha carbon as a nucleophile in an SN2 reaction and therefore can only remove protons (H+) that are on the outside of the molecule.
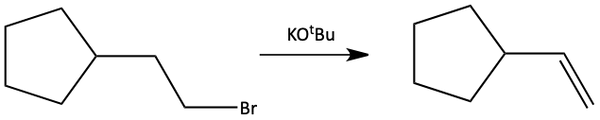
The SN2 reaction is prevented if the alpha carbon is crowded on the backside of the halide (like a tertiary alkyl halide). A backside attack could not occur. In this case, it doesn’t really matter if the base is large or small. As long as it is strong and aggressive, an E2 reaction will occur because the crowded substrate prevents the SN2 reaction.

17. Are the following bases more likely to be involved in E1 or E2 reactions?
a) water
b) CH3OH
c) OH-
d) (CH3)2N-
E2 Regiochemistry
Sometimes more than one beta carbon atom has a hydrogen atom that can be removed in the E2 reaction. Notice that we always remove the proton from a beta carbon, never the alpha carbon or farther away than the beta carbon, like gamma, etc. The reason for this is that the electrons need to fall between the beta and alpha carbons to make a π-bond.

The Saytzeff product is more stable than the Hofmann product. The Saytzeff product is the one with the most carbon groups attached to the alkene carbons. The Hofmann product has fewer carbon groups attached to the alkene carbons. All alkenes have four groups on them. Look at those four groups to see how many of them are carbon groups.


If the Saytzeff product is more stable, why would we ever get more of the Hofmann product? If a smaller base, like HO-, CH3O-, EtO- is used, the more stable Saytzeff product is made. But, if the Hofmann product is desired, a very large base like t-butoxide, tBuO- is used. A very big, bulky base like t-butoxide cannot reach the blue, interior protons. It can only reach the outside red beta protons. This leads to the Hofmann product.

18. For each of the following compounds, draw the Saytzeff and Hofmann products made following an E2 reaction.
a)

b)

c)

d) Circle the product in part (a) of this problem if KOtBu was used as the base for the elimination.
e) Circle the product in part (b) of this problem if NaOCH3 was used as the base for the elimination.
E2 Stereochemistry
In order for an E2 reaction to occur, the beta proton that is extracted by the base and the leaving group must be in a coplanar arrangement. The sp3 hybrid orbitals turn into p-orbitals and need to overlap to form the π-bond. The overlap can be syn-coplanar or anti-coplanar (anti-periplanar). Syn-coplanar is when the beta hydrogen and the leaving group have a dihedral angle of 0° and anti-coplanar has a dihedral angle of 180°.

Anti-coplanar (anti-periplanar) is preferred because the negatively charged incoming base is farther away from the negatively charged leaving group. You can also see in the Newman projection that anti-coplanar is in a more stable staggered conformation while syn-coplanar is in an unstable eclipsed conformation.
Anti-coplanar (anti-periplanar) is preferred.

Anti-periplanar E2 mechanism
Syn-coplanar can also happen, but it is not as preferred because the molecule must be in the higher energy eclipsed form.
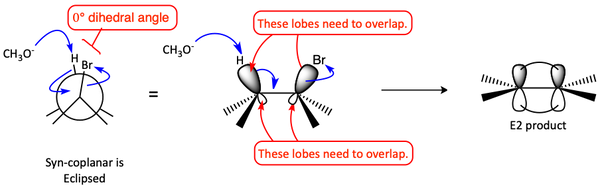
Syn-coplanar E2 mechanism
Typically, the syn-coplanar arrangement does not occur unless it is forced and the anti-coplanar arrangement cannot be obtained. In the following example, because of the bicyclic ring structure, only the syn-coplanar arrangement can be obtained with a dihedral angle of 0°. The red hydrogen atom and the red bromine atom are syn-coplanar to each other. The molecule cannot rotate to become anti-coplanar.

On cyclohexane chairs, the anti-coplanar formation can often be made. When 1-bromo-3-methylcyclohexane is attacked by a base, it can do an E2 reaction. In the lowest energy chair conformation on the left, there are no blue beta hydrogen atoms that are coplanar to the red bromine leaving group. Look closely until you convince yourself that they are not coplanar. But, if a chair flip occurs, now two hydrogen atoms (in red) are anti-coplanar to the bromine. The red hydrogen atoms and the bromine are in a 1,2-diaxial configuration. This is the only way to get a coplanar relationship in a cyclohexane chair.

Stereospecific
Because the anti-coplanar arrangement is preferred, that arrangement is most often formed. The E2 mechanism is also concerted (all happening at once), so there is no time for the molecule to rotate. The E2 mechanism can be considered a stereospecific reaction. One stereoisomer is made preferentially over another. In this example, we end up with the methyl groups on the same side as each other because that is how they started in the anti-coplanar arrangement.

19. Are the following reactions more likely to occur via the E1 or E2 mechanism (or neither)?
a)

b)

c)

Answers
17. a) E1 b) E1 c) E2 d) E2
18.

19.
a) E2 b) E1 c) Neither, poor leaving group